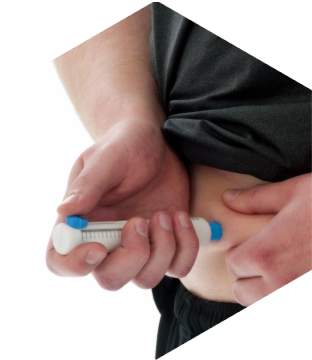
UniPen For BioTech & Pharma
UniPen.A single use, therapeutic agnostic injection safety device
By leveraging a therapeutic agnostic design, UniPen strips away the pain-points of device development and can reduce some of the large R&D expenses required for producing an injectable product.

Devices are Important
- An improved injection experience leads to increased satisfaction and increased adherence
- Devices are key to product differentiation and market competitiveness

Off the Shelf Solution.
- Developed with a target to reduce up-front monetary burden of launching your therapeutic product
- No need to defer device integration until after product launch

Designed to Reduce Integration Hurdles
- UniPen is an accessory to ISO Standard prefilled syringes
- As an accessory to the ISO Standard prefilled syringe, UniPen’s design is truly off-the-shelf, meaning no new development may be required for your product

Reduced Per Unit Expense
- Decrease per unit manufacturing cost compared to autoinjectors
- Decrease per unit assembly cost compared to autoinjectors
UniPenOff-the-shelf
Technical Design Characteristics
Primary Container:
- ISO Standard 1mL, long, staked-needle, glass prefilled syringe
- Needle length: ½ inch
Device modifications can accommodate variations to these requirements such as needle length and syringe volumes but are not offered as an off-the-shelf option.
CAUTION – Investigational device. Limited by Federal law to investigational use.
We Look To Ensure All Patients Are Given The Opportunity To Love Life
For more information on how Love Lifesciences’ UniPen can be integrated with your therapeutics.